
Cargando...
Que puis-je faire ?
226297 materialEducativo
textoFiltroFichatipo de documento Article de Wikipedia DbpediaThing
À propos de cette ressource...
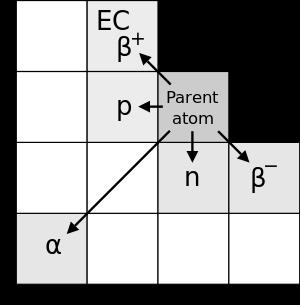
Contenu exclusif pour les membres de
Mira un ejemplo de lo que te pierdes
Catégories:
Étiquettes:
Fecha publicación: 20.4.2015
Que se passe t’il ? Inscrivez-vous ou lancer session
Si ya eres usuario, Inicia sesión
Ajouter à Didactalia Arrastra el botón a la barra de marcadores del navegador y comparte tus contenidos preferidos. Más info...
Commenter
0